Abstract
INTRODUCTION:
Pediatric inspired regimens in adolescent young adults and adults have improved outcomes in acute lymphoblastic leukemia (ALL). CALGB 10403 was prospectively tested in patients 17-39 years and achieved a 3-year event free survival of 59%. In 2007, we reported outcomes after incorporating peg-asparaginase (PEG) dosed at 2000mg/m in induction for patients aged 17- 55 years. This was well tolerated and achieved a complete remission (CR) rate of 96%. In 2014, we reported experience with the use of multiple doses of PEG (CCG-1882) for newly diagnosed ALL adult patients achieving a 7-year overall survival (OS) of 51%.
Since then, the USC ALL regimen consisting of 2 induction phases, was further modified with a goal of maintaining good outcomes and improving compliance and toxicities. Fractionated doses of cytarabine were changed to a single dose, the methotrexate dose of 1g/ m2 (2.5 g/m2 if T cell) given D1,15 in intensification phases was changed to 3g/ m2 (B and T cell) given in single doses, and consolidation was increased to six cycles allowing for PEG holidays.
Moreover, the approval and incorporation of novel agents such as blinatumomab and
inotuzumab also changed outcomes in ALL. Therefore, this study reports an update of
outcomes since further modification of the USC ALL pediatric-based regimen in the era of
novel agents.
METHODS:
This is a retrospective review which included adults aged 18 years with newly diagnosed Philadelphia negative ALL (2016-2020) treated at the USC/Norris Comprehensive Cancer Center
and the Los Angeles County Hospital (LAC). Primary objectives were over-all survival (OS),
event-free survival (EFS), disease-free survival (DFS) at 3 years. Secondary outcomes
were complete remission/complete remission with incomplete recovery (CR/CRi) and
minimal residual disease (MRD) by flow cytometry rates. OS, DFS, EFS were reported through
Kaplan Meier curves and Log-rank tests. Two-sided p value ≤0.05 was significant.
RESULTS:
121 patients with Ph-negative ALL were reviewed (49.6% Ph-negative B-ALL,
23.9% Ph-like B-ALL, 0.8% B cell lymphoblastic leukemia (LBL), 9.1% T cell ALL, 4.1% T cell
LBL, 4.1% early T-cell, 5.8% mixed phenotype acute leukemia (MPAL). Median age at
diagnosis was 39.3 years. There were 71.9% Hispanic, 15.7% White, 9.9% Asian, 2.5% African
Americans. 57.9% males, 42.1% females. In terms of risk stratification, the majority were unfavorable at 54.6% and 37.8% unknown. Median BMI was 29.6 kg/ m2. 52.5% had hepatic steatosis and 5% had CNS disease pre-induction. 68.7% received PEG during induction 1, and 67.9% during induction 2.
Post-induction 1, 83.5% patients achieved CR/Cri with 49% MRD negativity by flow.
Post-induction 2, 75% achieved CR/Cri with 57.1% MRD negativity by flow. 26.4% received blinatumomab but not allo-HSCT. 20% received blinatumomab and underwent allo-HSCT.
34.7% received blinatumomab for MRD flow positivity, median 2 cycles, only 5.8% received inotuzumab, 35.5% underwent allogenic stem cell transplant. 28.9% and 9.1% had any relapse and refractory disease, respectively. Mortality rate was at 14.9%.
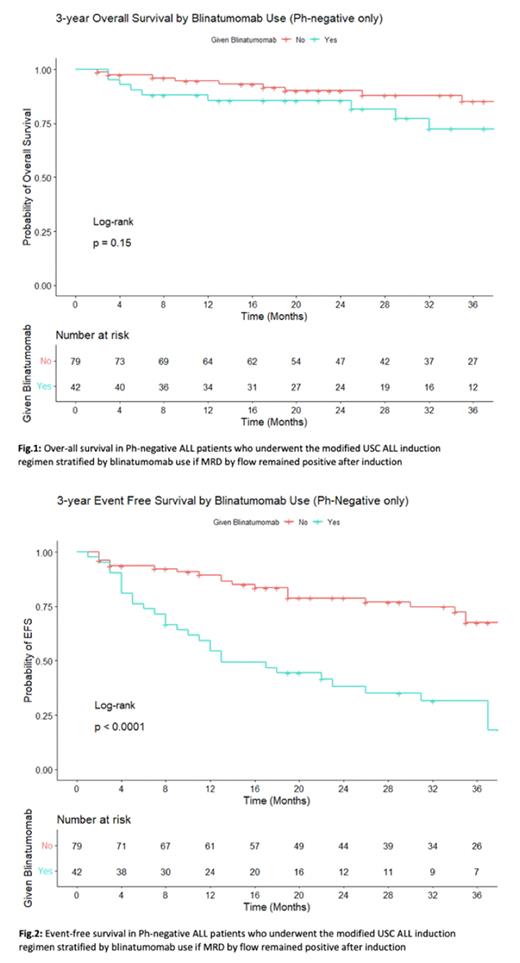
3y OS was at 80.7%, 3y EFS at 54.3% and 3y DFS 61.3%. When stratified by MRD flow negativity after induction 1 and 2, 3y OS was 83.7% vs 91.1% (p=0.55), 3y DFS was 68% vs 64.1%(p=0.98) and 3y EFS was 68% vs 59.5% (p=0.84). When stratified by blinatumomab use, 3y OS 72.5% vs 85.1% (p=0.15), 3y EFS was 31.6% vs 67.5% (p=0.001), 3y DFS was 40.1% vs 73.1% (p=0.001) (Fig 1-2).
Use of PEG resulted in grade 3/4 toxicities including hypersensitivity (4.1%),
transaminitis (21.5%), pancreatitis (5.4%), hypertriglyceridemia (49.5%), hypofibrinogenemia (45.5%), hyperbilirubinemia (21.5%) and thrombosis (16.5%).
CONCLUSIONS:
The modified pediatric-based USC ALL induction regimen using PEG maintained a high 3y OS at 85.3% and appreciable 3y EFS at 54.3% and 3y DFS at 61.3%, with tolerable toxicities in an era of novel agents like blinatumomab. This compares favorably with the original USC ALL regimen, CALGB 10407 and GRAALL 2005.
Of note, 3y EFS and 3y DFS were significantly lower in those who underwent blinatumomab since these patients were MRD flow positive and lacked deeper remissions, but 3y OS (72.5%) remained high regardless, even though 26.4% who received blinatumomab were not able to go for allo-HSCT. This suggests the beneficial effect of blinatumomab. Further follow-up is necessary to provide a more robust and mature survival data.
Disclosures
Chaudhary:Oncotartis: Consultancy; Pancella: Consultancy; Angeles Therapeutics: Consultancy, Current equity holder in private company; Moderna: Current equity holder in publicly-traded company; Celldex: Current equity holder in publicly-traded company; TCR2: Current equity holder in publicly-traded company; Allogene: Current equity holder in publicly-traded company; Athelas: Consultancy. Douer:Adaptive Biotechnologies: Speakers Bureau; Servier: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Yaghmour:USC: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal